In the realm of medical technology, ExoAtlet stands out as a pioneering company dedicated to enhancing the quality of life for individuals with mobility impairments.
Los Angeles, CA, California, United States – July 17, 2024 —

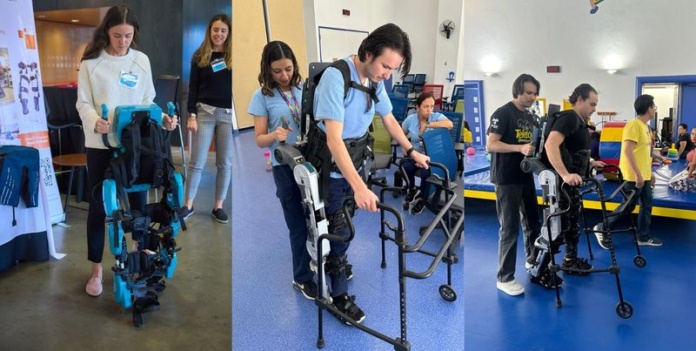
In the realm of medical technology, ExoAtlet stands out as a pioneering company dedicated to enhancing the quality of life for individuals with mobility impairments. Specializing in the development of exoskeletons, ExoAtlet aims to revolutionize rehabilitation and provide newfound independence to those affected by spinal cord injuries, stroke, and other neurological conditions.
ExoAtlet’s reach is rapidly expanding, with significant advancements in the Americas. Recently, the company opened an office in Los Angeles, USA, marking a strategic move to expand into the American market. This new headquarters will facilitate closer partnerships and streamline distribution channels across the continent.
In the United States, ExoAtlet has formed a key distributorship partnership with Neuro Rehab Recovery, a leading distributor of rehabilitation technology in the country. ExoAtlet has also collaborated with renowned rehabilitation hospitals, Kessler Foundation and Good Shepherd Rehabilitation. This collaboration will spearhead clinical trials for ExoAtlet’s pediatric exoskeleton, further cementing the company’s presence in the American medical landscape. With plans for FDA approval of their pediatric device in Q1 of 2025, ExoAtlet is poised to introduce pediatric exoskeletons to the US market, further advancing rehabilitation possibilities.
In Mexico, ExoAtlet has partnered with Tecno Logica Mexicana, the largest distributor of rehabilitation technology in the country. Additionally, ExoAtlet has collaborated with Fundación Teletón, a non-profit organization dedicated to serving people with disabilities, cancer, and autism. Fundación Teletón’s Children’s System (SIT), the largest private child rehabilitation system globally with 24 centers in Mexico, has integrated ExoAtlet’s pediatric and adult exoskeletons at their headquarters in CRIT State of Mexico, enhancing their comprehensive care offerings. Looking forward, the company aims to have COFEPRIS approval for their exoskeletons in Q1 of 2025, solidifying their role in the Mexican market.
South America also marks a significant expansion for ExoAtlet. The company has established a distributor partnership in Brazil and is collaborating with Dr. Linamara, a prominent Brazilian physician and professor specializing in physical and rehabilitation medicine. This collaboration aims to provide Brazilians access to ExoAtlet’s exoskeletons, with an exoskeleton placed at Rede de Reabilitação Lucy Montoro, a network of advanced rehabilitation centers in São Paulo.
Looking ahead, ExoAtlet continues to push the boundaries of what is possible in medical robotics. The company is investing in research and development to further enhance the capabilities of their exoskeletons, with the goal of making them more accessible to a broader range of patients. Future advancements may include improvements in battery life, increased range of motion, and integration with other therapeutic technologies. Through strategic partnerships, technological advancements, and a commitment to improving patient outcomes, ExoAtlet is shaping a future where mobility limitations are overcome with dignity and independence.
Contact Info:
Name: Kong Keun Cho
Email: Send Email
Organization: ExoAtlet
Address: Los Angeles, CA, California, United States
Phone: 1-213-440-3789
Website: https://www.exoatlet.com
Release ID: 89135482
In case of identifying any errors, concerns, or inconsistencies within the content shared in this press release that necessitate action or if you require assistance with a press release takedown, we strongly urge you to notify us promptly by contacting [email protected] (it is important to note that this email is the authorized channel for such matters, sending multiple emails to multiple addresses does not necessarily help expedite your request). Our expert team is committed to addressing your concerns within 8 hours by taking necessary actions diligently to rectify any identified issues or supporting you with the removal process. Delivering accurate and reliable information remains our top priority.